Abstract
Efficient metabolism in bioengineered tissues requires a robust vascular system to provide healthy microenvironments to the cells and stroma. Such networks form spontaneously during embryogenesis from randomly distributed endothelial cells. There is a need to bioengineer endothelial cells so that network formation and operation is optimal for synthetic tissues. This work introduces a computational model that simulates de novo vascular development and assesses the effectiveness of the network in delivering nutrients and extracting waste from tissue. A genetic algorithm was employed to identify parameter values of the vaculogenesis model that lead to the most efficient and robust vascular structures. These parameter values control the behavior of cell-level mechanisms such as chemotaxis and adhesion. These studies demonstrate that genetic algorithms are effective at identifying model parameters that lead to near-optimal networks. This work suggests that computational modeling and optimization approaches may improve the effectiveness of engineered tissues by suggesting target cellular mechanisms for modification.
Introduction
Multicellular organisms depend on vascular systems for nutrient delivery and waste removal. These vascular networks are formed either through vasculogenesis, a biological process in which scattered vessel precursor cells self-organize to create new networks or through angiogenesis, in which new vessels sprout from the existing vessels.
Both vasculogenesis and angiogenesis are driven primarily by chemotaxis, a mechanism in which cells move in response to a chemical gradient, along with cell-cell adhesion. While many questions remain, progress in understanding and exploiting both vasculogenesis and angiogenesis is being made from a bioengineering perspective. Dahl et al. successfully implanted tissue-engineered vascular grafts in baboons and dogs. Melero-Martin et al. showed that robust development of functional vascular networks is possible in vivo.
With additional research in this area, bioengineered cells could be used to form functional vascular networks to create a useful delivery mechanism in synthetic tissues. However, to apply bioengineering approaches to vascular cells, genetic targets need to be identified that when modified, improve the effectiveness of the vascular systems that emerge. Given the vast number of possible targets, a method is required that could assist engineers in identifying those genetic targets.
This paper presents a proof-of-concept genetic algorithm approach to solving this problem. The genetic algorithm acts to modify a computational model of vascular network development embedded within a cell cultivation environment. The search space of the optimization are the values of model parameters which control the mechanisms of the vascular cells such as chemotaxis. Changing these parameters modifies the behavior of individual cells which ultimately influence the spatial organization of the emergent vascular network. To evaluate the quality of this network, the model simulates its operation by determining fluid flow through each vessel, and subsequent nutrient delivery to the cultured cells. The returned fitness value quantifies the total metabolic activity of culture by using bioengineered microbial cells and measuring the total product produced.
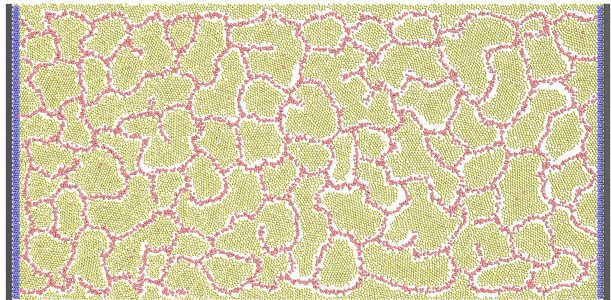 Figure 1. A sample cell culture simulation including producer microbial cells in yellow and endothelial-like
cells forming a vascular network in red.
Figure 1. A sample cell culture simulation including producer microbial cells in yellow and endothelial-like
cells forming a vascular network in red.
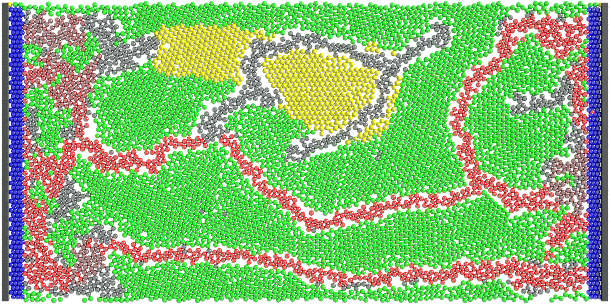 Figure 2. A sample active cell culture simulation including active producer microbial cells in green, inactive
producer microbial cells in yellow and endothelial-like cells forming a vascular network in shades of red;
higher flow vascular cell branches are depicted darker while lighter vascular cells show branches with lower
flow. The microbial cells which do not receive sufficient nutrients and do not have their waste and products
removed remain inactive as shown in the picture.
Figure 2. A sample active cell culture simulation including active producer microbial cells in green, inactive
producer microbial cells in yellow and endothelial-like cells forming a vascular network in shades of red;
higher flow vascular cell branches are depicted darker while lighter vascular cells show branches with lower
flow. The microbial cells which do not receive sufficient nutrients and do not have their waste and products
removed remain inactive as shown in the picture.
Genetic Algorithm Method
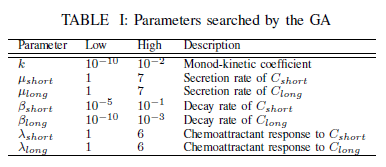
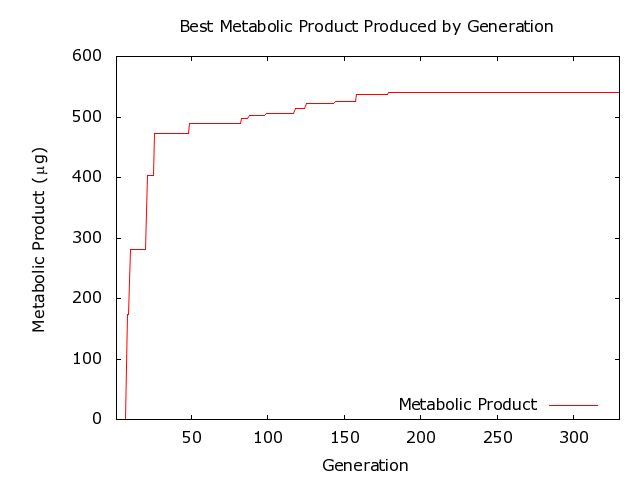
The self organizing vascular model has seven parameters with unknown values (Table 1). The best fit for these parameters was found using a genetic algorithm from the Java Genetic Algorithms Package [4]. The seven parameters are used as genes in individual chromosomes. The initial population had a size of three and was constructed by random selection of parameter values from the ranges shown in Table II. As the population evolves during runs of the genetic algorithm, each individual is evaluated using the parameter values encoded in the genes used to run the vascular model. The resulting vascular network is then evaluated, and the fitness is assigned based on the metabolic production. Next, genetic operators were run on the population followed by a re-evaluation of the population that now contained new individuals. Finally, selection was applied to obtain the new population for the next round of evolution. Crossover and mutation operators were included in the genetic algorithm, with the crossover operator applied to 35% of the population per generation and the mutation operator was applied to each gene with a probability of 1/12. An elitist ranking selector cloned the top %90 of the population, with the remaining 10% of the population filled with the same individuals in the order of fitness. The results presented in this paper were obtained using 1000 steps of evolution.
Results
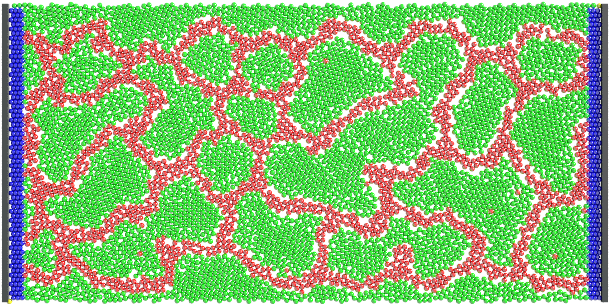 Figure 3. Product distribution of the best vascular network of endothelial-like cells that our algorithm has found in 1000
iterations.
Figure 3. Product distribution of the best vascular network of endothelial-like cells that our algorithm has found in 1000
iterations.
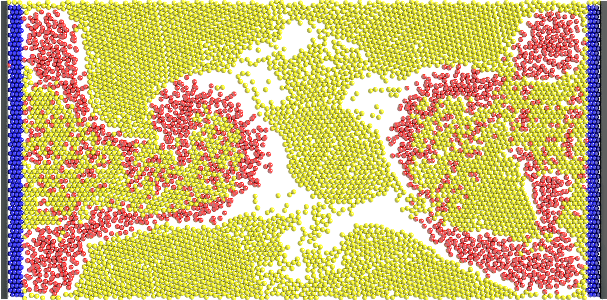 Figure 4. Sample results of the GA during its evolution. These results show unsuccessful attempts of the GA at creating a
consistent vascular network for the flow of nutrients and products
Figure 4. Sample results of the GA during its evolution. These results show unsuccessful attempts of the GA at creating a
consistent vascular network for the flow of nutrients and products
Conclusion
This study presents an attempt to optimize a vascular network of endothelial-like cells using genetic algorithm.
This work has shown that genetic algorithms are sufficiently powerful to optimize complex biological systems, even when there is a non-linear relationship between the parameters and the fitness function. This method can be applied to models of different biological systems that consider alternative objectives.
References
[1] “‘Exploiting Self-Organization in Bioengineered Systems: A Computational Approach’ by Delin Davis.” [Online]. Available: http://digitalcommons.usu.edu/etd/4914/. [Accessed: 04-Dec-2016]. [2] “iDynoMiCS: next-generation individual-based modelling of biofilms - Lardon - 2011 - Environmental Microbiology - Wiley Online Library.” [Online]. Available: http://onlinelibrary.wiley.com/wol1/doi/10.1111/j.1462-2920.2011.02414.x/citedby. [Accessed: 04-Dec-2016]. [3] “JGAP: Java Genetic Algorithms Package.” [Online]. Available: http://jgap.sourceforge.net/. [Accessed: 04-Dec-2016]. [4] “MOEA Framework, a Java library for multiobjective evolutionary algorithms.” [Online]. Available: http://moeaframework.org/. [Accessed: 04-Dec-2016].